The Challenges of Adopting Transcatheter Valve Devices in the United States with Dr. Ziyad Hijazi
By Adam Pick on November 22, 2013
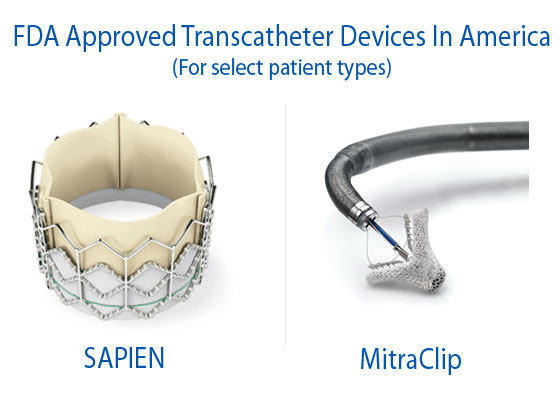
During the past 24 months, the Food & Drug Administration (FDA) has approved two transcatheter valve therapies — the SAPIEN and the MitraClip — for select patient populations in the United States. While these FDA approvals represent significant progress in the adoption of transcatheter valve technologies, I’ve met with several physicians and patients who continue to be frustrated by the relatively slow path by which next generation devices becomes available in America.
To learn more about the challenges and opportunities of adopting transcatheter valve therapy in the United States, I recently interviewed Dr. Ziyad Hijazi, a leading interventional cardiologist, from Rush Medical Center in Chicago, Illinois. Here are the highlights of my conversation with Dr. Hijazi.
I hope this video helped you learn more about the approval process of transcatheter valve devices in the United States. I would like to thank Dr. Ziyad Hijazi for taking the time to share his clinical experiences and research with our patient community.
Video Transcript of the Interview with Dr. Ziyad Hijazi
For those members of our community who are hearing impaired, I have provided a written transcript of this video with Dr. Hijazi below for your review.
Dr. Ziyad Hijazi: I am an interventional cardiologist dealing with congenital and structural heart disease. I’ve been in practice for over 20-some years, and I practice at Rush University Medical Center in Chicago, Illinois. What really attracted me to transcatheter valve therapy is the vision I’ve seen a few years where we are heading with interventional cardiology. Many, many invasive techniques where you can put a valve without cracking the chest.
During my career from 1990, when I finished my training, on average, I do personally about 200 cardiac catheterizations a year. This is only in my own institution. In addition to that, of course, all the cases I do traveling to the Middle East, to China, to South America, and all over the world, so thousands of cases. The major advantages of a transcatheter valve therapy is really intuitive You don’t have to crack the chest open. You go from the groin most of the time. Some of my colleagues using other values, they may go from the neck or just underneath the clavicle, and the patient’s recovery is phenomenal.
Like, nowadays, the differences are becoming less and less between an interventional cardiologist and a cardiac surgeon because many of the surgeons nowadays are learning the interventional techniques because most of the procedures now we are doing are minimally invasive, and the newer techniques, what we call the hybrid, is collaboration between the interventional cardiologist and the cardiac surgeons.
The approval a process in the United States is different than any other county in the world. They need to prove safety of the product and efficacy of the product. Proving something to be safe and effective may take years. In contrast, outside the United States, particularly Europe, they only require safety, which is short. You can do a study in about one or two months, prove that it is safe, it does not kill the patient, and you get it approved, and that’s why it takes longer in the United States for us to see the products that our colleagues across the pond already are using.
It is very frustrating for me as a clinician knowing that there is technology out there that I cannot provide my patients, this technology, and I’ve actually had a number of patients travel with me abroad because the devices that I wanted to use in the US were not available. I’m going to give you an example of a patient that I had here in Chicago. She was 91 years of age. She needed a transcatheter aortic valve replacement (TAVR) procedure, and for her to have the valve in the US, she had to enroll in a trial, and the trial, of course, is 50/50. The FDA wants us to compare the new therapy, the new valve, against the standard therapy, which is open heart surgery. So the computer assigns you 50 – you have 50% chance of being assigned to the new therapy, or 50% chance assigned to the conventional therapy. It’s good odds but for a patient, if you are selected to the conventional therapy, you got zero percent.
So, I called my colleague in Canada where the value is being provided for every patient. She flew there, she had her procedure, and she came back to Chicago for follow-up, and this is one example. We’ve sent patients to Europe for the same thing because they did not want to enroll in the trial and have only a 50% chance of being selected for the new therapy.
I believe that the best thing that can happen to accelerate the approval process for novel technology is maybe for the FDA to lower the criteria or lower the standards. What are they looking for? For example, we know that the transcatheter values, they work. So instead of come and do a brand new trial all over and compare it to conventional, let’s do a registry. A registry will take, let’s say 100 or 200 or 500 patients and offer them directly this therapy and follow them for a certain time and report on the results So you don’t have to go through the randomization process where you still commit patients to conventional therapy.
For example, now the only commercially available valve requires a large catheter, and many patients die from vascular complications. In my opinion, in this day and age, there is no need for a patient to die from a vascular complication because there are valves available outside the US that require half the size of the catheter in the US, so why subject my patients to this knowing that these values already have been approved outside the US with excellent results.
Again, many thanks to Dr. Hijazi for taking the time to educate our community about the adoption of transcatheter valve therapy!
Keep on tickin!
Adam