Special Technology Update: FDA Approves First Transcatheter Aortic Valve Replacement Therapy For “Inoperable” Patients
By Adam Pick on November 3, 2011
Big, big, big, biggggggg news everybody!
I just learned that the Edwards SAPIEN Aortic Valve has received FDA approval for “inoperable” patients. On the heels of this exciting announcement, there are several implications for patients and caregivers that should be considered.
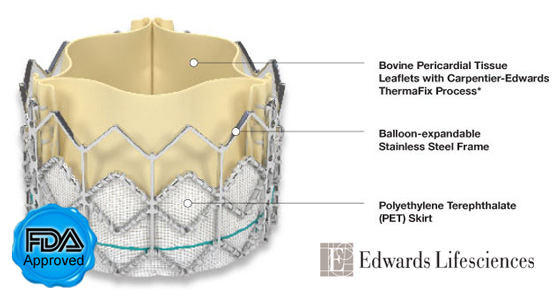
Edwards SAPIEN Heart Valve Replacement
First… This is the first time that a transcatheter therapy has been approved by the FDA for aortic valve treatment. Using SAPIEN, physicians deploy a catheter-based delivery system to replace a diseased aortic valve without any incision to the patient’s sternum or ribs.
Second… It must be emphasized that the Edwards SAPIEN valve is only intended for patients that are “inoperable”. Said differently, the FDA approved the SAPIEN for patients who are NOT eligible for open-heart surgery. The “inoperable” category includes patients with aortic valve stenosis and other ailments who are turned away from surgical treatment because it is unlikely they will survive the operation. To learn more about stenotic valves, click here.
Reflecting on the SAPIEN announcement, Dr. David Homes, a cardiologist at The Mayo Clinic, noted that the SAPIEN valve is a “game changer” for inoperable patients, many of whom are in their 80’s with medical conditions like diabetes, emphysema and liver disease.
Third… The SAPIEN valve, which is made of cow tissue, polyester and a stainless steel mesh, is intended for patients with aortic stenosis only. The device is not indicated for patients with aortic regurgitation or a bicuspid aortic valve.
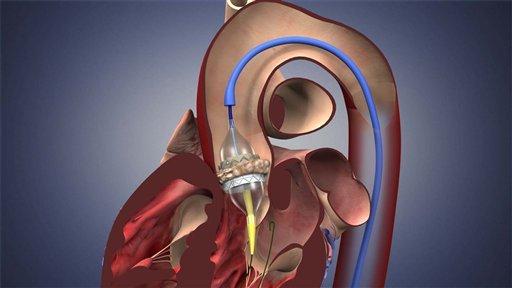
SAPIEN Aortic Heart Valve Replacement Implant Procedure
Fourth… Like most surgical procedures, the Edwards SAPIEN valve implant is not without risk. We must remind ourselves that this is a new technology that can and will be improved over time. During my recent conversation with Dr. Lars Svensson, Director of the Aorta Program and Quality at The Cleveland Clinic, he noted that transcatheter aortic valve implantations (TAVI) during the PARTNER trial did result in higher incidences of stroke. Doctor Svensson also noted that the clinicians and Edwards Lifesciences, the manufacturer of the SAPIEN, were working to decrease the stroke rate with strategies including filtering systems.
Fifth… I believe we need to offer Edwards Lifesciences a mighty “Congratulations!” on this important day. From what I understand, Edwards has spent an extraordinary amount of time and money to develop this technology. I am hopeful the Edwards’ team, which is led by Mike Mussallem, is taking the time to celebrate this transformational event for heart valve therapy in the United States.
“This day marks an important milestone for inoperable American patients who have long been awaiting a therapeutic option for the often debilitating symptoms associated with severe aortic stenosis,” said Michael Mussallem, Edwards CEO.
If you did not know, aortic stenosis is an often-overlooked and under-diagnosed disease that is deadly. According to reports, aortic stenosis kills 50% of patients within two years after the onset of symptoms. Today, thanks to Edwards’ commitment and pursuit of healthy heart valves, physicians may begin to treat a select, patient population that deserves the attention, the care and the right to live.
Sixth… Edwards is not alone in this accomplishment. Throughout the development of the SAPIEN valve, Edwards has relied upon and benefited from the clinicians – both surgeons and cardiologists – who have supported this effort. Personally, I would like to thank Dr. Smith, Dr. Stewart, Dr. Svensson, Dr. Roselli, Dr. McCarthy and so many others who have shared their clinical updates about the SAPIEN valve with our community over the years.
The medical technology that impacts heart valve therapy continues to amaze me. That said, I will continue to monitor such revolutionary advances, including the SAPIEN, in the future. To instantly receive my latest updates, you can subscribe to my complimentary newsletter by clicking here.
Keep on tickin!
Adam
P.S. If you are curious, reports estimate that about 300,000 people suffer from the degeneration of the aortic valve in the United States. According to reports, about 20,000 new patients will be eligible to receive a SAPIEN heart valve here in the United States on an annual basis.
|
Cheryl says on November 6th, 2011 at 8:52 pm |
|
Adam I am confused by your assertion that the Sapien valve is the first transcatheter valve approved by the FDA. I thought the Melody pulmonary valve was approved by the FDA back in 2010, long before the Sapien. My 13 year old son just recently received a Melody valve and it was a miraculous thing! I believe transcatheter valves are definitely a game changer as Dr. Homes said. Eventually, a percutaneous delivery will be the first choice for all valve replacements. As you know, all tissue valves wear out and must be replaced. My son’s next valve will have to be surgically placed because of the larger size he will need by the time the current one wears out. Maybe the one after that can be another Melody valve (we can hope). Or with a little luck medical science will have developed something even better! As the parent of a child born with a CHD, I would really like to see more of that part of the valve replacement community represented on your blog. There are many parents out there who have children receiving prosthetic heart valves who have no idea what to expect. Congratulations to Edwards Life Sciences on their accomplishment! |
 |
|
Adam Pick says on November 6th, 2011 at 9:00 pm |
|
Hi Cheryl, Thanks soooooo much for the catch. I goofed by not clarifying the statement relative to the aortic valve. I just edited it appropriately. So glad to hear all is going well with your son! Keep on tickin! |
 |
|
Norma says on March 26th, 2012 at 9:08 am |
|
My 79 y/o mom needs to have this (if she qualifies). I say it is a no-brainer (though it is not me having to decide). She has severe lung disease and this is her only option. |
 |
|
Toni says on June 13th, 2012 at 2:28 pm |
|
I appreciate this information Adam. Question: Are you aware of any work in the field that includes the transcatheter procedure to replace a biosynthetic aortic valve, or is it solely for native valve replacement? Thanks! |
 |
|
sandra chaplin says on June 25th, 2013 at 3:07 pm |
|
my Mom who is 87 had an angioplasty last week. she has been in icu since then . now that her blood pressure has stabilized, and her oxygen is regulated (has copd) we will go forward with rehab and if she meets criteria, then a TAVR is suggested by the hospital (JFK in florida) i am a bit apprehensive as they have done 45 of these procedures and the high risk of stoke associated with these procedures. what is the per centage of stroke results and are there any other risks involved? please send more info also. |
 |
|
Dr. Amit Kumar Chaurasia says on January 10th, 2019 at 4:58 am |
|
Heart Valve Therapy in gurgaon is one of the top hospitals in India. Follow the Heart Valve Therapy blog to stay up-to-date on the latest medical breakthroughs, services and treatment options offered by Dr. Amit Kumar Chaurasia. He is a dedicated interventional cardiologist with experience in both adult, pediatric and peripheral interventions. He is capable of performing TAVI, left atrial appendage closures, renal artery denervation apart from complex coronary and pediatric structural heart disease interventions. He also performs carotid and aorto-iliac interventions including intervention for aortic aneurysm and dissections. |
 |