Big News! FDA Approves SAPIEN 3 For Mitral & Aortic “Valve-In-Valve” Replacement!
Written By: Adam Pick, Patient Advocate, Author & Website Founder
Medical Expert: James Wudel, MD
Page Last Updated: May 13, 2025
In case you missed it…. There is some BIG NEWS to report. Last week, the SAPIEN 3 received FDA approval for mitral and aortic “Valve-In-Valve” replacement for high-risk patients. As you might remember, we’ve been talking about the wonderful possibilities of Valve-in-Valve techniques for years with doctors, like Dr. Eric Roselli, and patients, like Jesse McBride.
Needless to say, this is VERY exciting for patients with valve disease. We wanted to learn more about this development. That said, we connected with Dr. James Wudel. So you know, Dr. Wudel has been very involved with Valve-In-Valve procedures. In addition to being a proctor for this procedure, Dr. Wudel is also a great guy who has successfully treated many members in our community. Here are the highlights from my exchange with Dr. Wudel.

1. What is the significance of this Valve-in-Valve FDA Approval for patients?
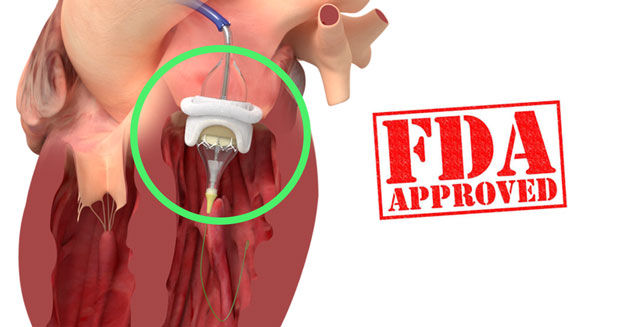
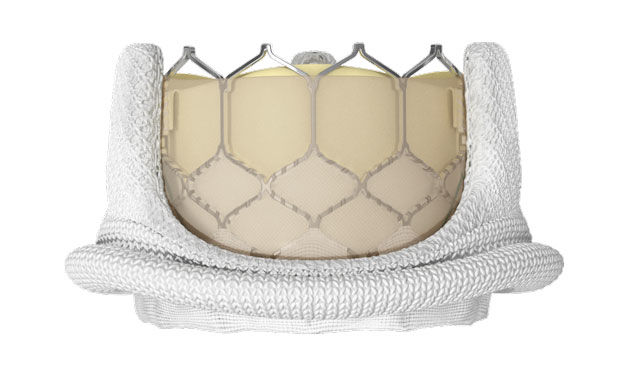
The FDA has approved the use of the Edwards Lifesciences SAPIEN 3 Transcatheter Heart Valve (THV) to treat dysfunctional/degenerated bioprosthetic valves in the aortic and mitral position. It’s important to note that this is the first transcatheter heart valve in the United States to receive this designation — for both aortic and mitral valves. Previously, other transcatheter devices were approved for just the aortic valve.
This Valve-in-Valve (ViV) designation allows for patients with “worn out” tissue mitral valve implants to be treated less invasively, yet effectively, by placing the SAPIEN 3 inside the old tissue valve.
2. Does this FDA approval transform your approach to valve therapy? If so, how?
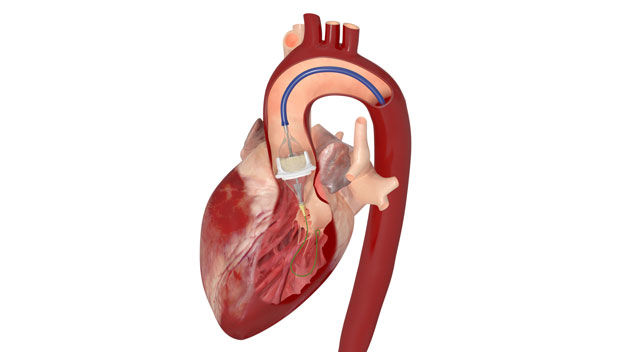
There are many patients that have received great benefit from their tissue mitral valve replacement over the years. However, we know these valves do not have the same durability as tissue aortic valves. So, it is not uncommon for elderly patients to have degenerated tissue mitral valves and no option except repeat heart surgery to replace the valve. The Edwards THV will provide a new, significantly less invasive treatment for these patients.
As the procedure can frequently be done using a groin vessel approach, the recovery is greatly improved. Secondly, this may further lower the age threshold to implant a tissue valve versus a mechanical valve in the mitral position for younger patients (should mitral valve repair not be possible).
3. What’s your personal experience with the Valve-in-Valve procedure?
These patients tend to have complex medical problems in addition to their degenerated tissue heart valve. The newly approved, less invasive, mitral Valve-in-Valve procedure has had excellent results in real-world scenarios for patients who otherwise face repeat “high-risk” heart surgery.
4. What is your advice for patients considering a Valve-in-Valve procedure?
I would recommend that any patient needing a replacement of a degenerated tissue heart valve to meet with their doctor and learn their options. These procedures are best performed using a “Heart Team” approach involving cardiologists and surgeons with significant experience in transcatheter, minimally invasive, and new imaging therapies for valvular heart disease.
Many thanks to Dr. Jim Wudel for taking the time to share his clinical experiences and research specific to Valve-in-Valve procedures with our community. We would also like to congratulate the Edwards Lifesciences team on this significant achievement. Thanks to the fantastic teamwork of doctors, like Dr. Wudel, and medical device manufacturers, like Edwards Lifesciences, the less invasive options for patients continue to expand.
Related Link:
Keep on tickin!
Adam
|
DONNIE says on June 16th, 2017 at 3:30 pm |
|
This is great news, my Aortic Valve is almost 15yo. |
 |
|
Larry Sparks says on June 16th, 2017 at 3:42 pm |
|
Do have any lung or heart issues after 15 years? Do you have a Bovine or mechanical Aortic valve ? |
 |
|
DONNIE says on June 16th, 2017 at 5:02 pm |
|
Just high blood pressure. Edwards Bovine Pericardial Valve. At doctors today have a little murmur, echo June 30. |
 |
|
Joan Pieroni says on June 16th, 2017 at 5:32 pm |
|
Donnie, can this be put inside your 15 yr old Valve? I have the same one and am hoping it will last 20 yrs |
 |
|
Adam says on June 16th, 2017 at 6:51 pm |
|
Donnie, Wow! 15 years? That’s great! Incredible! Fyi, I’m 11 years out from my aortic valve replacement. Maybe the SAPIEN could help both of us if we need a re-replacement? Keep on tickin’ Donnie! Adam |
 |
|
Adam says on June 16th, 2017 at 6:52 pm |
|
Great question Joan. Do you have a tissue valve as well? |
 |
|
Joan Pieroni says on June 16th, 2017 at 6:53 pm |
|
Yes the Edwards magna ease paracardial aortic valve |
 |
|
Sheila says on June 16th, 2017 at 8:04 pm |
|
My son was born with heart valve defect and had cardiac cath at 2months of age. Now he is 11 and he has severe aortic regurgitation. I am worried about open heart surgery to replace valve and was curious to know it would be an option for kids to do transcatheter. |
 |
|
Scott Barton says on June 19th, 2017 at 7:39 pm |
|
This is very good news for me personally. I have undergone three open heart surgeries at the Cleveland Clinic since 2007. The first two procedures were repairs and the third a replacement. My current bovine replacement valve is compromised due to some rare and unexpected clotting that damaged the leaflets. Obviously, a fourth surgery is high risk. I have spoken with my cardiologist in Cleveland and he indicated that I might be a candidate for this lower risk procedure. I will be heading to the Clinic in the near future for some additional testing and evaluation. I will keep you posted. |
 |
|
Pipsqweek says on July 10th, 2017 at 7:54 pm |
|
Adam,it is 3 in the morning here in Basel Switzerland,at 6.30 Dr Reuthebuch will with the Swiss Edwards backup will give me their latest “INSPIRAS RESILIA”. While not approved in America thanks to the FDA whose dubious decisions are predicated not on our health but for the profit of hospitals in America. |
 |
|
compassn says on July 11th, 2017 at 9:27 pm |
|
It was just approved by the FDA but won’t be available here in the US for about six months. |
 |