CoreValve Technology Gets European Approval
By Adam Pick on September 9, 2010
As technology for heart valve treatment rapidly advances, we continue to learn more about the roll-out of percutaneous devices for both heart valve repair and heart valve replacement. On Tuesday, Medtronic announced that regulators in the European Union have approved the latest version of its CoreValve heart system.
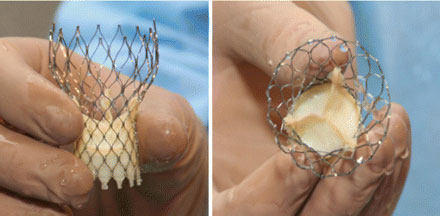
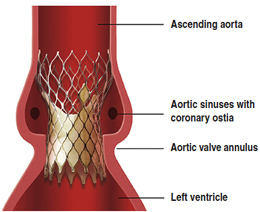
The CoreValve delivery system is designed to help surgeons replace a diseased aortic valve without removing the original valve and without open heart surgery. Instead, they put a new valve into a catheter and guide the catheter to the heart from an artery in the leg.
The CoreValve system was first approved in Europe in March 2007, but is not yet approved in the United States.
The newest version of the system includes AccuTrak technology, which Medtronic said will give surgeons greater control during implant procedures. The CoreValve system was indicated to treat patients with severe aortic valve stenosis without open-heart surgery.
I will continue to update this blog as both Medtronic and Edwards Lifesciences continue their clinical trials in anticipation of FDA approval — for the CoreValve and Sapien solutions — in the United States.
Specific to severe mitral valve regurgitation disorder, I’m also closely monitoring the data released by Abbott Laboratories for its MitraClip system for mitral valve repair.
Keep on tickin!
Adam
|
mark jansen says on September 9th, 2010 at 1:21 pm |
|
I am encouraged to see the rapid advances of the percutaneous devices and the CoreValve system can be a treatment option for those with severe aortic valve stenosis. Can this same device be used for a bicuspid valve with aortic regurgitation without stenosis? |
 |
|
jim says on September 9th, 2010 at 1:25 pm |
|
Hey Adam, can this device be used for regurgitant aortic valves as well? Jim |
 |
|
Adam Pick says on September 9th, 2010 at 5:04 pm |
|
To Mark and Jim, I’m running out the door right now but here are some quick responses to your questions. * The CoreValve is, unfortunately, not currently designed to treat aortic regurgitation. * The CoreValve is, unfortunately, not currently designed to treat bicuspid aortic valves. I’ll post a follow-up blog about this shortly. Thanks for the great questions! Adam |
 |
|
Joseph says on September 15th, 2010 at 6:48 am |
|
Dear Adam, This is certainly a procedure that I would be very much interested in considering. I am close, in fact I’m here ready to select a surgeon having had another echo done most recently. Although I have no symptoms, the valve is getting tighter. I’ve looked over Dr. Allan Stewarts site at Colombia and it states that he does this procedure; Transcatheter Aortic Valve replacement. How can this be if the procedure is not yet approved in this country? All the best, Joseph |
 |