Stroke Risk & Heart Valve Calcification: What Should Patients Know?
Written By: Adam Pick, Patient Advocate & Author
Medical Expert: Dr. Marc Gerdisch, Chief of Cardiac Surgery, Franciscan Health
Page Last Updated: June 3, 2025
For many patients, calcium build-up on heart valves is considered “Public Enemy No. 1” as calcified valves lead to stenosis, debilitating symptoms, heart failure and death. In addition, as we recently reported here, our community of patients and medical care providers are learning about potential risks of calcium supplements for older patients.
As a result, my inbox has lit up with great questions about calcium and heart valve disease. In particular, patients are concerned about whether-or-not calcium deposits on a diseased heart valve can “break off” and cause a stroke. To learn about calcified valves and stroke risk, we interviewed Dr. Marc Gerdisch, the Chief of Cardiac Surgery at Franciscan Health in Indianapolis, Indiana. Dr. Gerdisch is a heart valve expert who has performed more than 4,000 heart valve repair and replacement operations. In our community, Dr. Gerdisch has successfully treated over 120 patients including Dan Rhoden and Janelle Hurst.
Key Learnings About Heart Valve Calcification & Stroke Risk
Important learnings shared by Dr. Gerdisch in our interview include:
- Calcified heart valves are stiff and narrowed which blocks blood flow through the valve, the heart and, ultimately, the body.
- Calcification is a function of metabolic changes in the leaflets and valve structure. The changes can occur due to atherosclerotic changes, inflammation, and abnormal cell development. As Dr. Gerdisch states, “When we’re talking about calcium in the body, we’re talking about something similar to bone. It’s getting deposited, not in an organized fashion like a bone, but getting deposited in this inflamed space that induces the stiffening of the leaflets. Overtime, they can become rock hard.”
- Risk factors that may lead to atherosclerosis (calcification) include smoking, diabetes and hypertension (high blood pressure). According to Dr. Gerdisch, genetic pre-disposition plays a critical role in the development of heart valve calcification. In addition, patients who undergo radiation to their chest may struggle with aortic and mitral stenosis. Rheumatic disease is another source of calcified heart valves.
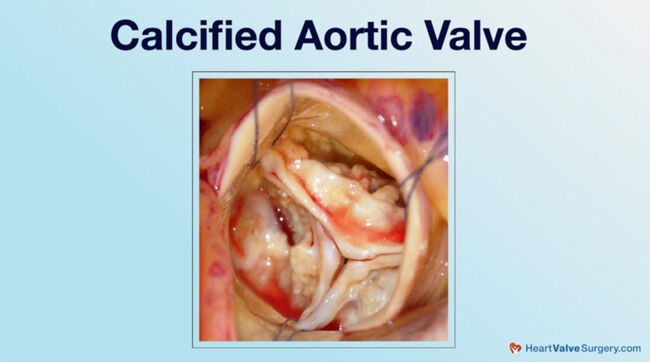
- Age-related calcification is a newer consideration for patients and their medical teams. Dr. Gerdisch states, “Folks develop age-related calcification, both in the aortic valve, the leaflets, and the annulus, which is where the valve leaflets attach, but also in the mitral valve. We’re seeing more of that, especially in women. That’s heavy calcification in the annulus, the ring around the valve. Very difficult to manage and treat because that calcification can grow through the wall of a heart.”

- Specific to the risk of calcium “breaking off” a heart valve and causing a stroke (thromboembolism event), Dr. Gerdisch believes that is possible but very rare. “Another concern that people have, and it’s legitimate, is can that calcification break off? Can it mobilize and embolize?” states Dr. Gerdisch, “The answer is yes, but it is rare. It has always been fascinating to me when I look at a valve in the operating room, aortic or mitral, but especially aortic we see this in, and we just touch that valve and the calcium will just break off. You can’t believe that it doesn’t come off all the time.”
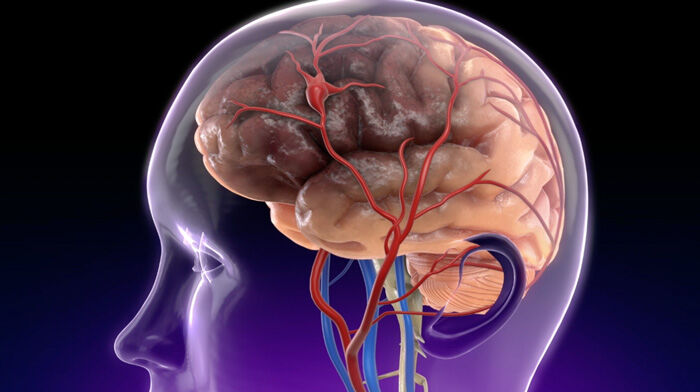
- Dr. Gerdisch is sensitive to any form of “mobile calcium” that is detected under echocardiography which is ultimately treated surgically or via transcatheter approaches. Dr. Gerdisch states, “If you had an echocardiogram and you see calcium that’s flipping around, then that’s a little bit more concerning. We will see at times filamentous or mobile calcium. The other important concern for us is that when we do something to the valve that we ensure that that calcium doesn’t go anywhere that it’s not supposed to go. It’s very straightforward when, for example, we replace a valve or work on a calcified valve directly in surgery with an open heart because we can see it all. We take the calcium out. When we do transcatheter interventions, especially when we do transcatheter aortic valve replacements (TAVR), we don’t have that luxury. We’ve learned that if we see anything mobile, then we should – we can protect the cerebral vasculature.”
- De-calcification surgical techniques are used by Dr. Gerdisch when possible to help patients retain their own tissue which is advantageous for long-term outcomes. Dr. Gerdisch states, “We’ll take a piece of calcium out and reconstruct the valve, do what we need to do to make the valve soft and working again because our goal still remains to leave the patient with their own tissue. Folks all know about this at some degree, but every time we can leave you with your own tissue, you’re better off. “
- Patients with calcified heart valves need to have thoughtful conversations about treatment approaches (repair versus replacement) specific to durability and quality-of-life. Should a patient and their medical team determine a heart valve replacement is required, a discussion of which valve to select – mechanical or tissue valve – is critical. While mechanical valves do not “re-calcify”, the patient is required to be on blood thinners for the balance of their lives. While tissue valves do not require blood thinners, the cow or pig tissue tends to start deteriorating upon implant. Dr. Gerdisch states, “Once you put that tissue valve in, it starts to deteriorate. Part of that deterioration is very similar to what’s happening to a native valve, inflammation, calcification.”
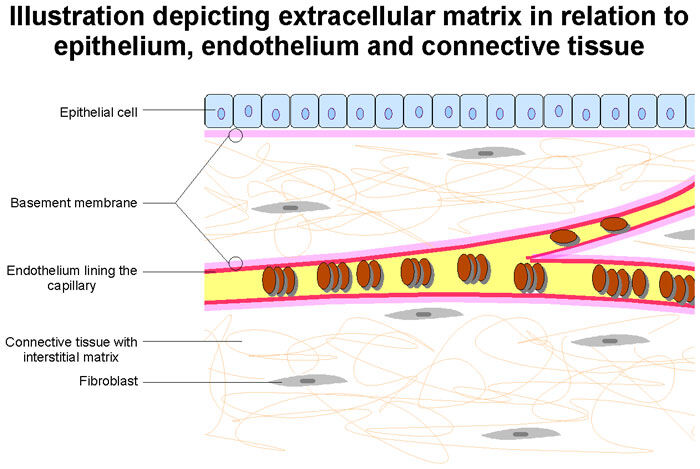
- Looking into the future… Dr. Gerdisch is currently researching-and-developing new forms of tissue for heart valve replacement devices. Dr. Gerdisch states, “The last thing I would add is tissue engineering, which we’ve been working on for 15 years. The past decade, we’ve made some real progress. We have a valve that is made of extracellular matrix, which that’s in the midst of an FDA study. Our goal there is to provide matrix for the patient to build their own tissue and hopefully have a native valve again. Probably it won’t be perfect, but it might get closer to something that will last a long time and behave like their own tissue.”
Many Thanks Dr. Gerdisch & Franciscan Health!
On behalf of our entire patient community, many thanks to Dr. Gerdisch for sharing his clinical experience and research with our community! Also, many thanks to the Franciscan Health team for taking such great care of heart valve patients.
Related links:
- Coronary Artery Disease & Heart Valve Surgery: Top 9 Facts
- Atrial Fibrillation: A Concern for Heart Valve Patients
- Surgeon Q&A: Will I Need Mitral Valve Surgery?
- Stroke Risk & TAVR: What Should Patients Know?
Keep on tickin!
Adam
P.S. For the hearing impaired members of our patient community, I have provided a written transcript of this video below.
Video Transcript:
Adam Pick: Hi, everybody. It’s Adam with heartvalvesurgery.com. This is a very important surgeon question and answer session all about heart valve calcification and stroke risk. I am thrilled to be joined by Dr. Marc Gerdisch who’s the chief of cardiac surgery at Franciscan Health in Indianapolis, Indiana. During his fantastic career, Dr. Gerdisch has performed over 6,000 cardiac procedures with more than 4,000 involving some form of heart valve repair or heart valve replacement. Dr. Gerdisch, you and I have known each other for a long time. I am very excited to learn from you in this very important conversation about heart valve calcification and stroke risk. Thanks for being with us today.
Dr. Gerdisch: Thanks, Adam. Yeah, it is an important conversation. It’s important for people to understand some of the background on calcification of heart valves.
Adam Pick: Yeah, and so for the patients, let’s start with an obvious question but incredibly relevant, which is what is a calcified heart valve?
Dr. Gerdisch: A calcified heart valve is any valve that stiffens with the development of calcium in it. People say, “Well, why is that happening? Am I taking too much calcium? Do I eat the wrong foods?” The answer is no. The calcification is there as a function of metabolic changes in the leaflets of the valves. Typically, when we’re talking about the leaflets, we’re talking about the aortic valve. The aortic valve is the valve for which the leaflets most commonly calcify.
It occurs as a function of a couple of things. First, there’s usually some injury to the leaflets. That injury can overlap with something we’ve talked about before which is atherosclerotic disease. When people have atherosclerosis, which is blood vessel disease, they can have the same process in the leaflet aortic valve. When there’s injury to those leaflets, it establishes inflammation. The inflammation then engenders the migration of small blood vessels into the leaflets, and then that delivers abnormal cells into the leaflets. Those cells can take different paths in their development and they can actually turn into osteoblast. Those are cells that make bone.
When we’re talking about calcium in the body, we’re talking about something similar to bone. It’s getting deposited, not in an organized fashion like a bone, but getting deposited in this inflamed space that induces the stiffening of the leaflets. Overtime, they can become rock hard.
How does the injury occur? The first one I talked about is atherosclerosis. There are things like smoking, diabetes, hypertension. Those are metabolic and physical stresses on the leaflets that can induce injury and then lead to inflammation and calcification. The other is turbulent flow. Folks who have bicuspid aortic valves, from the day they’re born, they have abnormal flow around the valve. Those valves then can be injured by that and then calcified, but that doesn’t really explain the whole thing because everybody that has a bicuspid valve doesn’t get a calcified valve. There are people with bicuspid valves that live their whole lives with that valve functioning well. There are people with bicuspid valves who never get any calcium in them. They just leak. They change in shape and then they leak and then we repair those. There are folks who get calcification in those bicuspid valves.
What’s the difference? The difference is genetics. Some people with bicuspid valves also have the gene elements that lead to the calcification or lend to calcification. That’s going to be true of people with normal valves, too. There’s going to be – there are a lot of people who have a genetic predisposition to calcifying their aortic valves. That’s getting sorted out in detail in very sophisticated ways, but we don’t have a way to cure it. There’s not a way to fix that. It’s similar to people who have genetic predisposition to coronary artery disease. There are people who have no other comorbidities but they have a genetic predisposition and they get blockages in their coronary blood vessels. There are people who have a genetic predisposition to getting calcification and thickening of their aortic valve leaflets.
Other types of injury include, for example, I see a lot of patients, relatively a lot of patients, who have had radiation to their chest. That radiation can cause injury to the aortic valve and then we have to treat that. We have to replace that valve. Radiation can be done when they’re quite young, and often it is. Often, they’re in their 20s or something, and then 20 years later, I’m taking care of their aortic valve or even their mitral valve. Mitral valves can be affected by radiation as well. Often, we’re dealing with replacing those valves. The other interesting overlap is that in those same patients, because the radiation is delivered to their chest, they can develop coronary artery disease, too. Radiation has changed. Radiation therapy has changed a lot in the last 10 to 15 years. Most of these patients were treated a long time ago.
Another one I should mention is rheumatic valve disease. Rheumatic valve disease is the consequence of having had structural when you were a child. It just so happens you didn’t get antibiotics. Those people develop an autoimmune response which then attacks their own valves. It can affect the mitral valve and/or the aortic valve. Today I saw a gal who’s got an aortic and mitral valve that both need to be replaced because they’ve become thickened, calcified, immobile. There’s classic changes that occur from rheumatic disease, but it includes calcification.
The other one that has become more prominent really in the past decade for us, that we’ve noticed anyway or we’re dealing with more, is that folks develop age-related calcification, both in the aortic valve, the leaflets, and the annulus, which is where the valve leaflets attach, but also in the mitral valve. We’re seeing more of that, especially in women. That’s heavy calcification in the annulus, the ring around the valve. Very difficult to manage and treat because that calcification can grow through the wall of a heart. When we have to replace those valves, we often have to take all that calcium out and reattach the upper to lower chambers, the atrium and the ventricle, and then put a new valve in.
You can see it’s a spectrum of disease. Not all calcifications are the same. Some of it involves more the leaflets. Some of it involves more the annulus, or where the leaflets attach. Probably the only thing that we can identify that is consistent that is not genetic is inflammation, so some type of injury and inflammation, and then genetic predisposition.
Adam Pick: Dr. Gerdisch, super helpful. Let’s move on and talk about the potential consequences of being predisposed or having calcium build up on valves. What can happen to patients?
Dr. Gerdisch: The consequences are multiple. First of all, when any calcium is identified in a valve. that’s a signal that there’s something going on. You want to look at the patient from a cardiometabolic standpoint. Is their diabetes managed? Is their blood pressure under control? Are there elevated inflammatory markers? Those could be measured. Before a patient has a problem, consequence, it could be a signal. When you see it on an echocardiogram, when we see it on a scan, we want to go after that. We want to look at the metabolic file of a patient.
The next thing, of course, is stenosis. If it starts to stiffen the leaflets and the leaflets don’t open or the calcium in the annulus and the mitral valve into the opening of the valve, then the valve becomes stenotic and then we’ve got a stenotic or obstructed valve. That has to be treated. Another concern that people have, and it’s legitimate, is can that calcification break off? Can it mobilize and embolize? The answer is yes, but it is rare. It has always been fascinating to me when I look at a valve in the operating room, aortic or mitral, but especially aortic we see this in, and we just touch that valve and the calcium will just break off. You can’t believe that it doesn’t come off all the time.
Now, in 30 years, I’ve seen many patients. I’ve seen patients who have no other reason for the stroke that they had other than the calcium that we could find in their leaflets. That doesn’t mean for sure that’s where it came from, but that’s what it looked like it came from. Those tend not to be big events. They tend to be small events. That means that maybe some little pieces can break off. I would have to caution people about getting alarmed about calcium in their valve and worrying about a stroke because it’s so unlikely but possible.
The calcifications would take different forms. If you had an echocardiogram and you see calcium that’s flipping around, then that’s a little bit more concerning. We will see at times filamentous or mobile calcium. That we become more concerned about. Calcium that’s just in one place that is affecting leaflet function but it hasn’t caused a real problem for the patient yet, that calcium the likelihood of it breaking off or causing a problem is very small.
The other important concern for us is that when we do something to the valve that we ensure that that calcium doesn’t go anywhere that it’s not supposed to go. It’s very straightforward when, for example, we replace a valve or work on a calcified valve directly in surgery with an open heart because we can see it all. We take the calcium out. We repair or replace the valve. We do what we need to do. We have control of that. When we do transcatheter interventions, especially when we do transcatheter aortic valve replacements, we don’t have that luxury. We’ve learned that if we see anything mobile, then we should – we can protect the cerebral vasculature.
If we see a risk, and that happens in folks that we see bulky calcification that might be mobile, in people who have bicuspid aortic valves that we’re treating transcatheter, which we don’t very often. We usually just do surgery for them but there are scenarios where we do bicuspid valve. Somebody that, for example, we’re doing valve. This is another category. This is somebody who’s had a previous valve implanted. Now it’s calcified. We’re going to manipulate it. We’re going to put a valve inside of that.
In all of those scenarios, we will actually go through the radial artery. We’ll put nets in under the blood vessels that feed the brain. Then we’ll do the procedure because we don’t want any of that calcium to get up to the brain. To recap, calcification in valves are in change and we should be looking at. Only when it’s clearly mobile is it something that someone should be concerned about on a follow-up echocardiogram or something. Certainly at the time of any intervention, we have to be sure that we have absolute control of it and we’ve done everything we can to prevent it from mobilizing.
Adam Pick: Dr. Gerdisch, thanks so much for sharing all this incredible information. I’m guessing patients might be wondering, if I have a predisposition for build up of calcium on my valves, if I have an operation to repair or replace it, is it likely that I’m just going to have build up again? If so, what could I potentially do to prevent a build up of calcium on the valves?
Dr. Gerdisch: That is such a great question. I’ve heard it before. Obviously, one of the interesting things about people with valve disease is that it usually isn’t a sudden problem. They study it a little bit. They learn a little bit. They’re curious then, like you just posed that great question, about the relationship between what caused their valve problem and then how it continues later in life. It is very much tailored to the particular patient. I do think it’s a worthwhile question for people to ask. It does come to some degree the judgement of the clinician as well as balancing the concerns of the patient.
The reason I tell you all of that is because, for example, when you repair a valve and you have to decalcify it, how aggressive should you be in that process? We do that. We do that in aortic and mitral valves where we’ll take a piece of calcium out and reconstruct the valve, do what we need to do to make the valve soft and working again because our goal still remains to leave the patient with their own tissue. Folks all know about this at some degree, but every time we can leave you with your own tissue, you’re better off. If we can give you normal functions, it is durable.
We will pursue that even with some calcification to a point because if we go too far, we’re left with a fair amount of damaged leaflet. That damaged area probably will stiffen again over time. We don’t know the exact time course. When we decalcify, though, we are leaving behind tissue that was calcified before and it probably will calcify again over time, but we don’t know exactly how long it took to calcify. We don’t know how long it will take in the future. We don’t have a magic pill to keep it from happening.
The other part of that is when we replace a valve. It’s part of the conversation of tissue valve versus mechanical valve. It is the thing that is being pursued in research and in some of the more novel technology to lessen the impact of that calcification, for example, in tissue valves. Mechanical valves never calcify. They open and close forever, but you have to take blood thinners for them. The conversation always becomes, do you want to be on blood thinners? Do you need to be on blood thinners? What is it like to be on a blood thinner? Versus a tissue valve that won’t require that but that has a clock on it. Once you put that valve in, it starts to deteriorate. Part of that deterioration is very similar to what’s happening to a native valve, inflammation, calcification.
There are two components to it in tissue valves, cow and pig tissue. One is just calcification by calcium attaching to moieties that are free attachment points on a leaflet. They’re actually these fatty components that the calcium can attach to. Part of one of the biggest projects in tissue technology is to cover those. They’re hidden by newer means of what we call anti-calcification. Some folks may have heard of the INSPIRIS valve, which is the latest iteration of the Edwards device that does have some promise with lowering the calcification, but we don’t have any long-term data on that. We can’t prove that that’s going to have that consequence.
The other component is just plain old immune response. We know that’s a foreign body and our body responds to it which is probably why they don’t last as long in younger people because they have strong immune responses. We don’t have a magic pill that will alleviate that problem, that will stop that calcification from occurring. The answer is that we have to have judgment with respect to how much calcium we might take on native valves. We have to be realistic about the durability of tissue valves that will have this Achilles heel aspect of calcification. I think folks should rest assured that all those opportunities exist and that a good clinician is going to figure out the best the patient.
Adam Pick: Dr. Gerdisch, thanks for bringing in the conversation about valve selection, tissue valve, mechanical valves. What role might tissue engineering have for heart valve patients?
Dr. Gerdisch: The last thing I would add is tissue engineering, which we’ve been working on for 15 years. The past decade, we’ve made some real progress. We have a valve that is made of extracellular matrix, which that’s in the midst of an FDA study. Our goal there is to provide matrix for the patient to build their own tissue and hopefully have a native valve again. Probably it won’t be perfect, but it might get closer to something that will last a long time and behave like their own tissue. We’re not the only people working in that. Tissue engineering is active in valve technology. I do think within the next decade we’re going to see some really cool stuff that’s going to change the way people think about prosthetic valves and replacement valves.
Adam Pick: Dr. Gerdisch, I love how you continue to innovate in the field of heart valve therapy. I can’t thank you enough on behalf of all the patients at heartvalvesurgery.com for helping us learn today about heart valve calcification and stroke risk. Thanks so much.






