Tech Update: Encouraging Data For Edwards’ SAPIEN Aortic Valve Replacement
By Adam Pick on March 26, 2012
For those of you monitoring the progress of the transformational Edwards SAPIEN Valve, which replaces the aortic valve in “non-operable” patients, more encouraging data was just released at the American College of Cardiology Conference in Chicago. The SAPIEN is the first catheter-based device, approved by the FDA, that replaces a valve suffering from aortic valve stenosis without any trauma to the patient’s sternum or ribs.
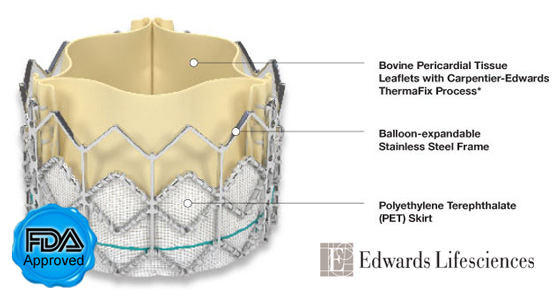
In the latest study results:
- The number of patients who died was almost identical regardless of whether they were treated with traditional surgery or SAPIEN. After 2 years, 33.9 percent of patients treated with with SAPIEN died compared with 35% of those getting open heart surgery; and
- The stroke risk, twice as high in the SAPIEN group one month after treatment, was less common in the ensuing months after non-invasive aortic valve replacement and ultimately not significantly higher than with surgery.
Reflecting on the study, Susheel Kodali, an interventional cardiologist and co-director of the Transcatheter Aortic Valve Program at Columbia University Medical Center in New York, stated, “We have established that it is equivalent to surgery in these high-risk patients… One of the big worries was stroke. This doesn’t put it to rest, but it alleviates a lot of concern. There has been no signal of any sign of valve failure, so that’s reassuring.”

Susheel Kodali, Interventional Cardiologist
However, it should be noted that patients treated with SAPIEN were more likely to develop leakage around the valve called paravalvular regurgitation, and those people were twice as likely to die, the study found. Patients treated with the Edwards valve experienced a type of leakage found in many studies of catheter-delivered valves. About 10% had moderate to severe leakage and 40% experienced mild leakage.
“We were comparable to surgery done by the best surgeons in the world,” Kodali said in an interview. “There aren’t going to be significant improvements in surgery. This was an early stage experience with the transcatheter valve. As we get more experience, better screen patients and develop newer devices, we’re going to improve outcomes.”
As always, I will continue to monitor these exciting technology announcements about next-generation heart valve therapies.
Keep on tickin!
Adam
|
Dave T says on March 26th, 2012 at 5:06 pm |
|
Great news! I was originally going to go with a mechanical valve, but my surgeon changed my mind. He said the bovine valves these days could last 15-20 years, and I wouldn’t need blood thinners which have both risks and lifestyle limitations. I make a living working with wood, so i get scratched and cut regularly…blood thinners would make life difficult to say the least. Anyway, he believes that in 15 years, trancatheter aortic valve replacements should be commonplace, so I may not require another sternotomy down the road…fingers crossed! |
 |













